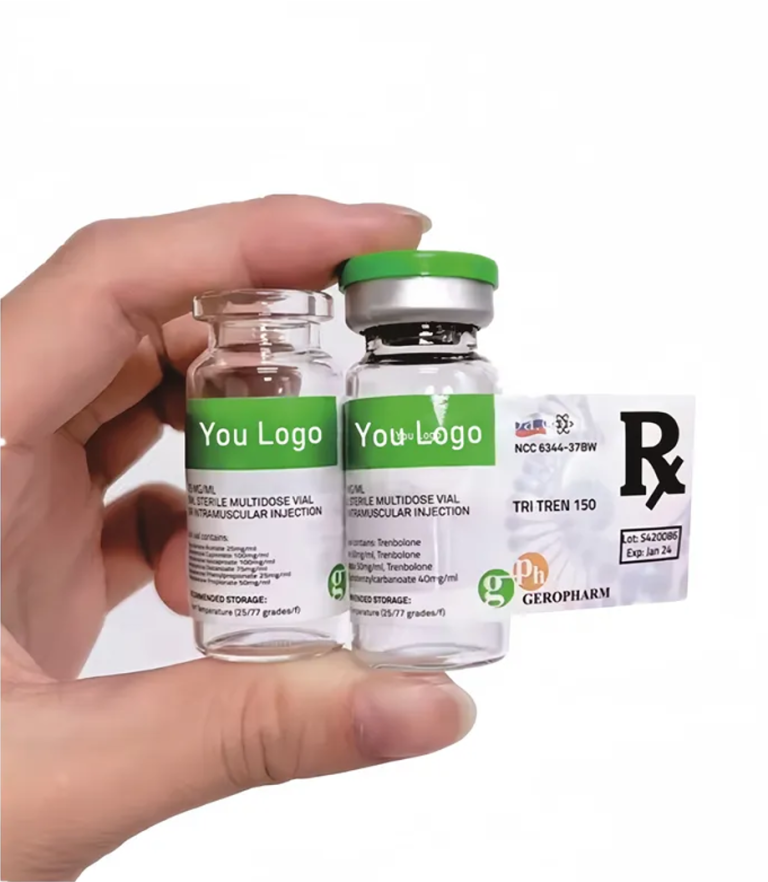
🔹 What are Medical Labels?
Medical labels refer to the information printed on the immediate packaging (such as bottles or ampoules) and outer packaging (like boxes) of pharmaceutical products.
They are not only the “ID card” of the medicine but also a critical bridge between regulatory compliance, healthcare providers, and end-users.
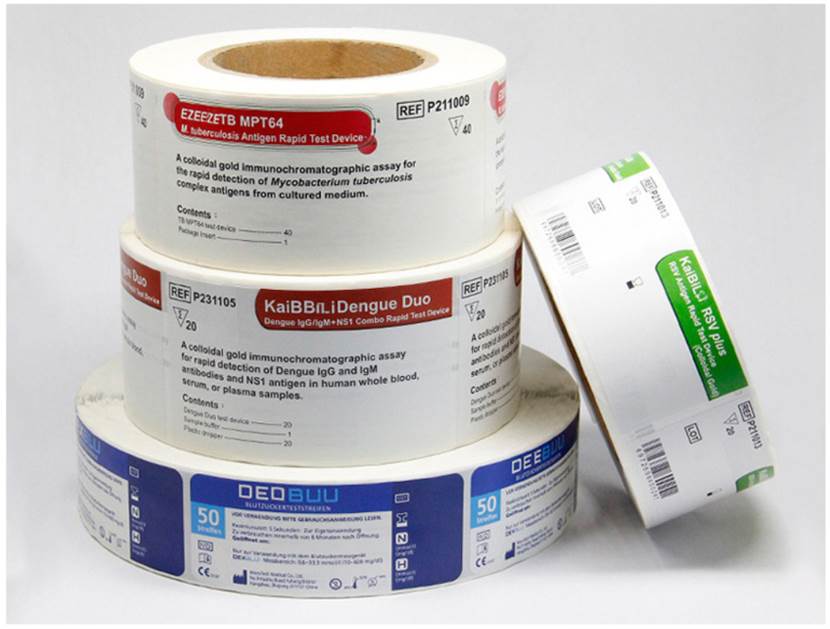
🔹 Why Are Medical Labels Important?
Medical labels are a mandatory part of drug marketing and sales. Their core role is to ensure public safety and proper usage. Here's why they matter:
🔍 Communicate critical information: Drug name, dosage, usage, expiration date, and contraindications help avoid misuse.
🛡 Protect patient safety: Warning messages and anti-counterfeiting designs reduce risks of overdose or mix-ups.
✅ Ensure regulatory compliance: Without compliant labels, drugs cannot be sold legally in markets like the EU or the USA.
🌍 Support global distribution: A compliant label enables smooth registration and acceptance across multiple countries.
🔹 What Information Should a Medical Label Contain?
- Outer Packaging must include:
- Product name, active ingredient(s), and strength
- Dosage form and content (e.g.“10 tablets/box”,“5ml/vial”)
- Route of administration (oral, injection, etc.)
- Warning statements (e.g., “Keep out of reach of children”, “Prescription only”)
- Expiry date (“EXP”), Batch number (“LOT”)
- Storage conditions
- Name and address of the marketing authorization holder
- Country of manufacture/registration
- Braille for the product name (required in EU)
- Immediate Packaging must include at minimum:
- Product name, dosage, batch number, expiry date, route of administration
👉 Note: Some products—such as radioactive drugs, small-vial vaccines, or traditional herbal medicines—may require additional warnings or declarations due to their specific nature and risks.
✅ Key Requirements for Medical Labels: Safety, Compliance, Accuracy
1. Clear, legible, and durable
Labels must be easy to understand and printed with durable materials that resist fading, smearing, or peeling.
2. Adaptable to special environments
🧊 Cold storage drugs: Labels must withstand low temperatures and condensation.
☢ Radioactive products: Labels should use UV- and corrosion-resistant materials.
✅ How to Customize Your Medical Labels?
Work with a professional label manufacturer to ensure full compliance and quality.
MC Package is a label manufacturer with 20 years of experience. Simply send us your requirements—including design files, drug type, label size, and preferred material—and we will provide a tailored labeling solution that ensures safety, compliance, and durability.

Our advantages:
- ✅ Regulatory compliance: Our designs follow FDA and EU labeling guidelines.
- 🔍 Enhanced readability: High-resolution print ensures clarity, even on small containers.
- 💧 Durability: Waterproof, UV-resistant, and chemical-resistant materials protect labels from damage.
- 🔐 Security features: Tamper-evident seals, serial numbers, and QR codes available to enhance traceability and product integrity.
📩 If you have labeling needs, please feel free to contact us. We offer both small-batch customization and large-scale production.